The FAS Lab is located in the beautiful city of Lisbon, Portugal. Our antibody engineering research group is affiliated with the Centre for Interdisciplinary Research in Animal Healthy (CIISA) at the Faculty of Veterinary Medicine, University of Lisbon. We apply rational design and molecular evolution approaches – such as in vivo phage display selections, functional screenings and next generation sequencing analysis – to develop future generations of therapeutic antibodies. Based upon this integrated approach, we aim to develop a new generation of “smart bomb antibodies” against challenging targets and unmet diseases. For this purpose, our lab is mainly working with antibody fragments (Fab and scFv) and single-domain antibodies (sdAbs) as therapeutic scaffolds and in the areas of infectious diseases, cancer and neurodegenerative disorders.
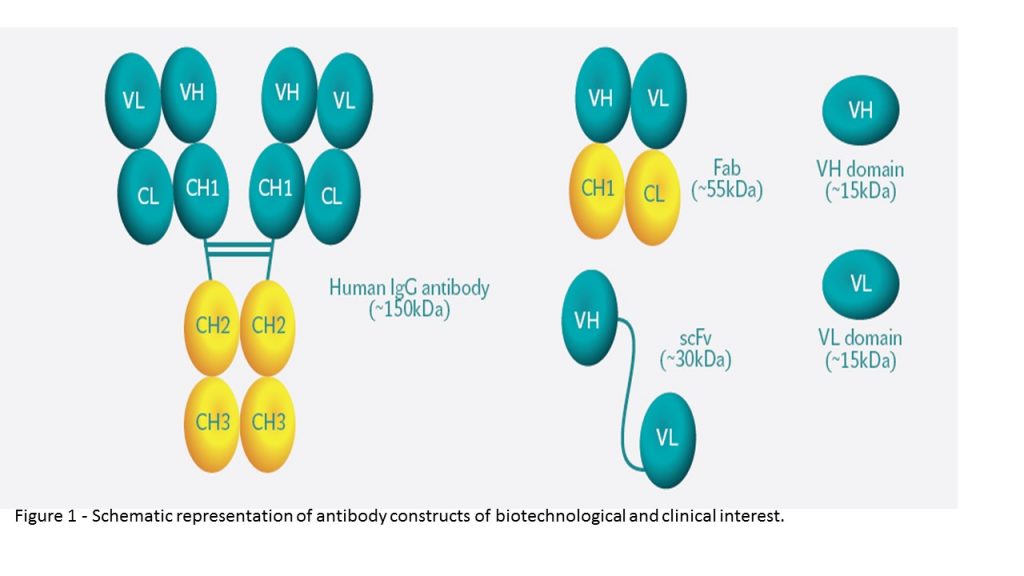
Most therapeutic Mabs consist of a full-length IgG molecule because of its predominance in human serum, importance for immune response, and excellent specificity characteristics. Nevertheless, although most marketed antibodies are comprised of a full-length IgG molecule, this conventional antibody format presents some drawbacks that limit their clinical use and there is a range of therapeutic applications in which other antibody formats may be more appropriate. First, due to high molecular weight (~150 kDa), IgG antibodies are known to penetrate poorly into densely packed tissues, to have an impaired reaching of difficult targets and to present a slow clearance rate. Second, inappropriate activation of Fc receptor-expressing cells sometimes leads to massive cytokine release with subsequent toxic effects. As such, with the advances of genetic engineering, smaller antibody molecules are being developed such as the antigen binding fragment (Fab) or the single-chain variable fragment (scFv) promoting the development of new recombinant molecules and antibody fragments with multiple applications (Figure 1).
Another promising alternative to conventional IgGs are single-domain antibodies (sdAbs). sdAbs are the smallest functional antigen-binding fragments of an antibody that can be isolated from conventional IgGs (e.g., from human, murine, or rabbit) or obtained from naturally occurring antibodies devoid of light chain that were discovered in two types of organisms, the camelids (camels and llamas) and cartilaginous fish (wobbegong and nurse shark). Due to their small size, sdAbs present improved tissue penetration and are able to reach targets not easily accessible by conventional IgG molecules, such as enzyme active sites or canyons in receptors molecules. Moreover, such as Fab and scFv, sdAbs lack the Fc domain of a full IgG antibody, presenting a low nonspecific uptake in tissues that highly express Fc receptors. Additional important advantages include their high stability, low immunogenicity, and lower manufacturing cost. Furthermore, sdAbs can be easily attached to other proteins, peptides, small molecules, or nanoparticles by simple molecular biology or chemical procedures. The capacity to combine sdAbs with each other or with other protein domains and payloads enables the development of bispecific and bifunctional molecules which diversifies even more its range of therapeutic applications.
Taking into consideration all these advantages and properties, smaller antibody scaffolds are definitely a promising alternative to conventional antibodies for immunotherapy applications. Therefore, one of the main research lines in our lab has been the development of engineered andibody fragments (Fab and scFv) and sdAbs against different targets using a combination of techniques that include antibody library construction from diferent sources (rabbit, mouse, camel, shark and human) and types (immune, naïve or synthetic); in vitro and in vivo phage display selections; high-throughput screenings (HTS) and next generations sequencing analysis.
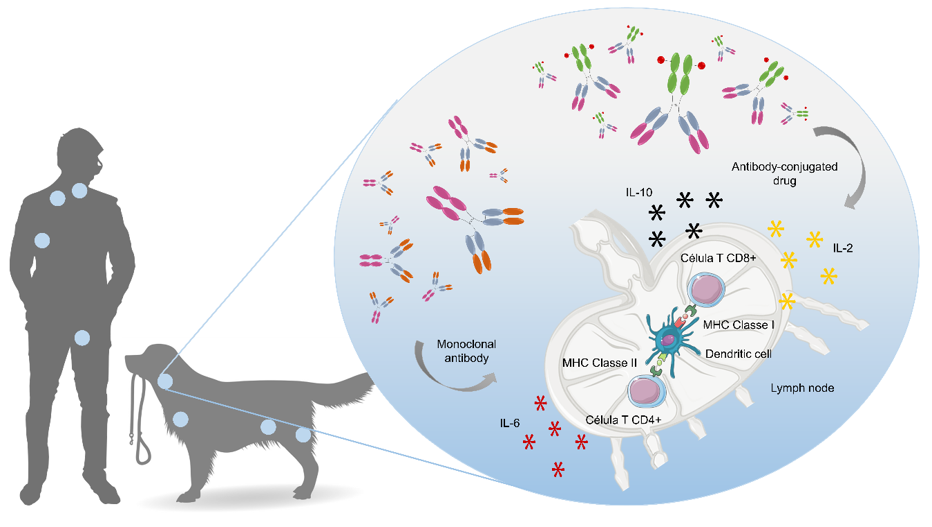
Cancer is among the leading causes of death worldwide, representing one of the most critical unmet medical need of today’s society. Our Lab aims to provide solutions that improve cancer patient’s quality of life and prolong lives. For that purpose, veterinary clinicians and non-clinical researchers from FASLab work together to expand their knowledge of the biology of neoplasms and their treatment. At the core of our oncology programme is the validation of the pet dog as an animal model for immunotherapeutic approaches in comparative medicine. Notably, FASLab offers an integrated drug discovery platform that maximize interdisciplinary cooperation and leverage commonalities across humans and dogs, for the development of novel immunotherapies against non-Hodgkin lymphoma and glioblastoma, benefiting both species. Currently, we are investigating new immunotherapies that mobilize the patient’s own immune system and antibody-based drug delivery systems that selectively direct potent payloads to the site of the disease. For that purpose, we are exploring novel antibody selections and the identification of potent payloads to the develop a next generation antibody drug conjugates and immunolipossomes for cancer treatment.
Ongoing projects:
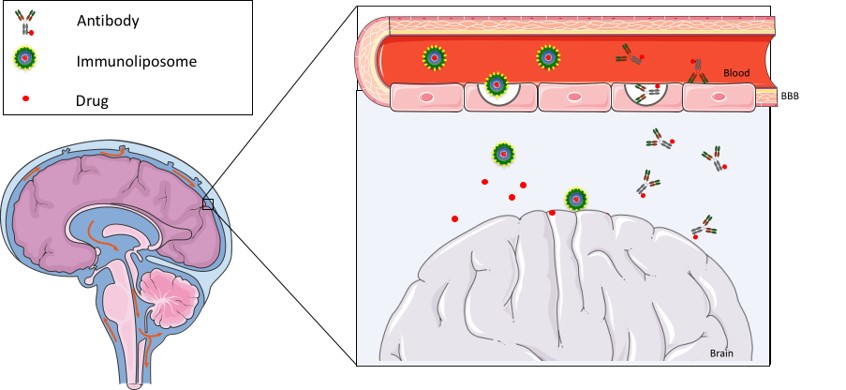
Central nervous system (CNS) disorders, such as Alzheimer’s, Parkinson’s and brain tumors, affect up to 1 billion people worldwide and its incidence continues to rise, posting enormous burden on societies with aging population. Despite the progress made toward understanding these diseases, few effective treatments and no cures are currently available. In fact, a major issue in the development of new and effective CNS therapies is the limiting effect of the blood–brain barrier (BBB), a physical and selective metabolic barrier between the brain and the systemic circulation. One approach to overcome the BBB bottleneck is to target endogenous BBB transport systems, and to develop CNS drug delivery strategies that take advantage of these natural portals of entry into the brain. As such, there has been a strong interest in the development of recombinant antibodies targeting BBB receptors. Some of these antibodies specifically transmigrate the BBB and have been tested as vectors to deliver drugs into the brain. However, by relying on receptors that are not brain specific, such as transferrin and insulin receptors, these antibodies can lead to mis-targeting effects.
Therefore, there is an urgent need to discover novel BBB endogenous receptors that can provide a more selectively delivery into the brain. Within this context, our lab is exploring novel antibody selections and the promising properties of single domain antibodies to develop highly specific BBB transmigrating antibodies that can be efficiently used for brain targeting and drug delivery across the BBB.
Ongoing projects:
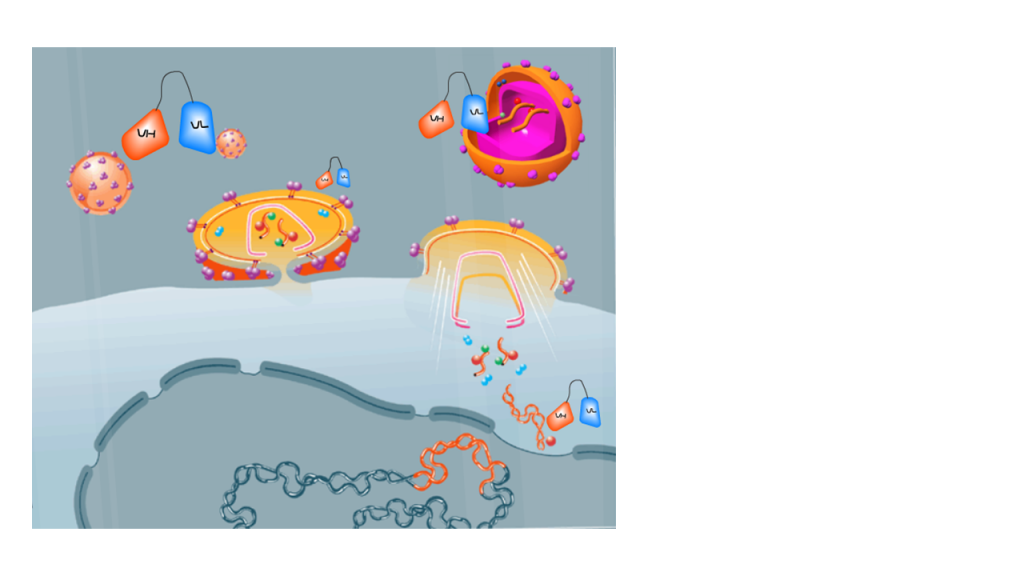
Infectious diseases are an important cause of morbidity and mortality around the world. With the advent of anti-viral and antibiotic resistance this field represents an urgent unmet need with a global demand for safer, more effective, anti-infective drugs. Our Lab aims to develop innovative pathogen-targeted approaches for HIV, Meningitis, Sepsis and antibiotic resistant bacteria. We are exploring a variety of new antibody constructs based on conjugates of antimicrobial peptides, antibiotics and drug-encapsulated liposomes. We are also focused in the development of sustainable and reproducible in vitro and in vivo models for testing anti-infective drugs.
Ongoing projects: